For more detail, please contact:
Dr. C. P. Paul
Convener, Incubation Centre, RRCAT
Phone: +91 731 248 8396
Mobile: +91 94256 66596
Shri Praveen K. Agrawal
Co-convener, Incubation Centre, RRCAT
Phone: +91 731 244 2404
Mobile: +91 94258 31055
Email: incubation[at]rrcat[dot]gov[dot]in
|
Events/ Happenings/ News-items
Electron Beam Sterilization of 630 cartons of BD Venflon Pro I.V. Cannula at ARPF, RRCAT, Indore
RRCAT received an order for electron beam sterilization of 630 cartons (individual devices 3.15 lakhs) of VenflonTM Pro I.V. Cannula at ARPF (Fig.-1) from M/s Becton Dickinson India Pvt. Ltd., Bawal, Rewari, Haryana. The product belongs to Risk Class-B and is used for blood/fluid transfusion. M/s Becton Dickinson (BD), is one of the largest global medical technology companies in the world.
The e-beam radiation processing was done during 03 to 07 Oct 2022 as per SOPs under the Quality Management System (QMS) applicable for the regulated medical device (ISO 11137, Medical Device Rules-2017 and ISO 13485). RRCAT successfully achieved the high quality levels required for e-beam sterilization, at par with international standards, and was audited through in-depth audit by third party agencies and the quality control division of M/s Becton Dickinson.
Quality control during the processing was done by keeping the machine parameters within permissible tolerance bands, continuous logging and verification of process data. In addition, reference dosimeters (alanine pallets) were used which were independently read and confirmed.
After successful inventory QC checks as per ARPF QMS requirements, the complete consignment was divided into 20 batches (32 cartons in each batch) and the traceability of each carton inside the facility was established by unique bar-code system. The non-irradiated and irradiated cartons have been stored in different, designated storage areas, Fig. - 2 (a & b) and identified using irreversible radiation indicator (turned red after irradiation from yellow before irradiation), Fig.-3 (a & b). The linac was operated at 9.3 MeV, 6 kW beam power (effective irradiation time 22 hr.). Fig. - 4 shows the critical process parameter during irradiation. The average minimum dose and maximum dose delivered to the cartons were, 29.5 kGy and 52.0 kGy which is within the specified range of M/s BD (range of 25 to 55 kGy). Performance Qualification (PQ) for the product was done earlier this year at ARPF.
The consignment has been dispatched to the customer after irradiation on 20th October 2022.
Read More...
 |
 |
 |
Fig.-1: BD IV cannula VenflonTM
|

Fig.-2 (a): Non-irradiated BD cartons stored in non-irradiated product storage area of ARPF
|

Fig.-2 (b): Irradiated BD cartons stored in ready to dispatch product storage area of ARPF
|

Fig.-3 (a): Non-irradiated carton barcode |

Fig.-3 (b): Irradiated carton barcode |
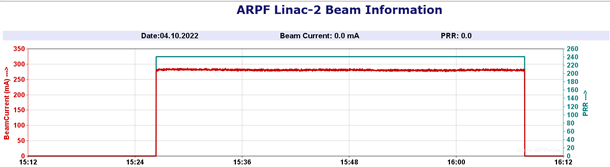 |
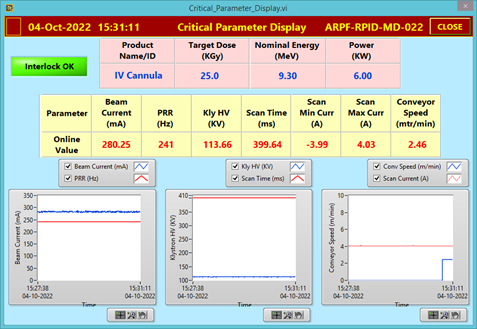
Fig.-4: Beam parameter of Linac while irradiation and critical parameter display |
Read less...
|























